TMEM106B focus
Previously unknown protein found in neurodegenerative disease tissue
For many neurodegenerative diseases, a common hallmark is harmful protein aggregation. A good example of this is the accumulation of the protein TDP-43, a DNA/RNA binding protein that pathogenically accumulates in the neurons of patients with frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Other well-studied pathogenic proteins include Tau and α-synuclein. These proteins become pathogenic through conformational change into fibrillar or multimeric species, acting to aggregate their native isoforms and convert them into pathological molecules.
Recently, a new protein has been discovered that highlights a whole new avenue for neurodegeneration research. Chang and colleagues (2022, Cell) were looking for the identity of a fibrillar protein found in FTD patient brain tissue, which they assumed was a form of TDP-43. Surprisingly, however, upon further investigation with cryo-EM and mass spectrometry, the sequence was revealed to be 120-253aa of TMEM106B. Two papers in Nature were also released around the same time confirming this finding, showing the fragment to be present in patients with a range of neurodegenerative disease tissue and in healthy aged brain tissue (Jiang et al. 2022, Nature; Schweighauser et al. 2022, Nature). TMEM106B is a lysosomal transmembrane protein that is also a known risk factor for neurodegenerative diseases (Van Deerlin et al. 2010, Nature Genetics); thus, TMEM106B is no stranger to this field. Taken together, these findings will shift the focus of neuroscience research across the globe, with scientists racing to determine the importance of the protein in disease.
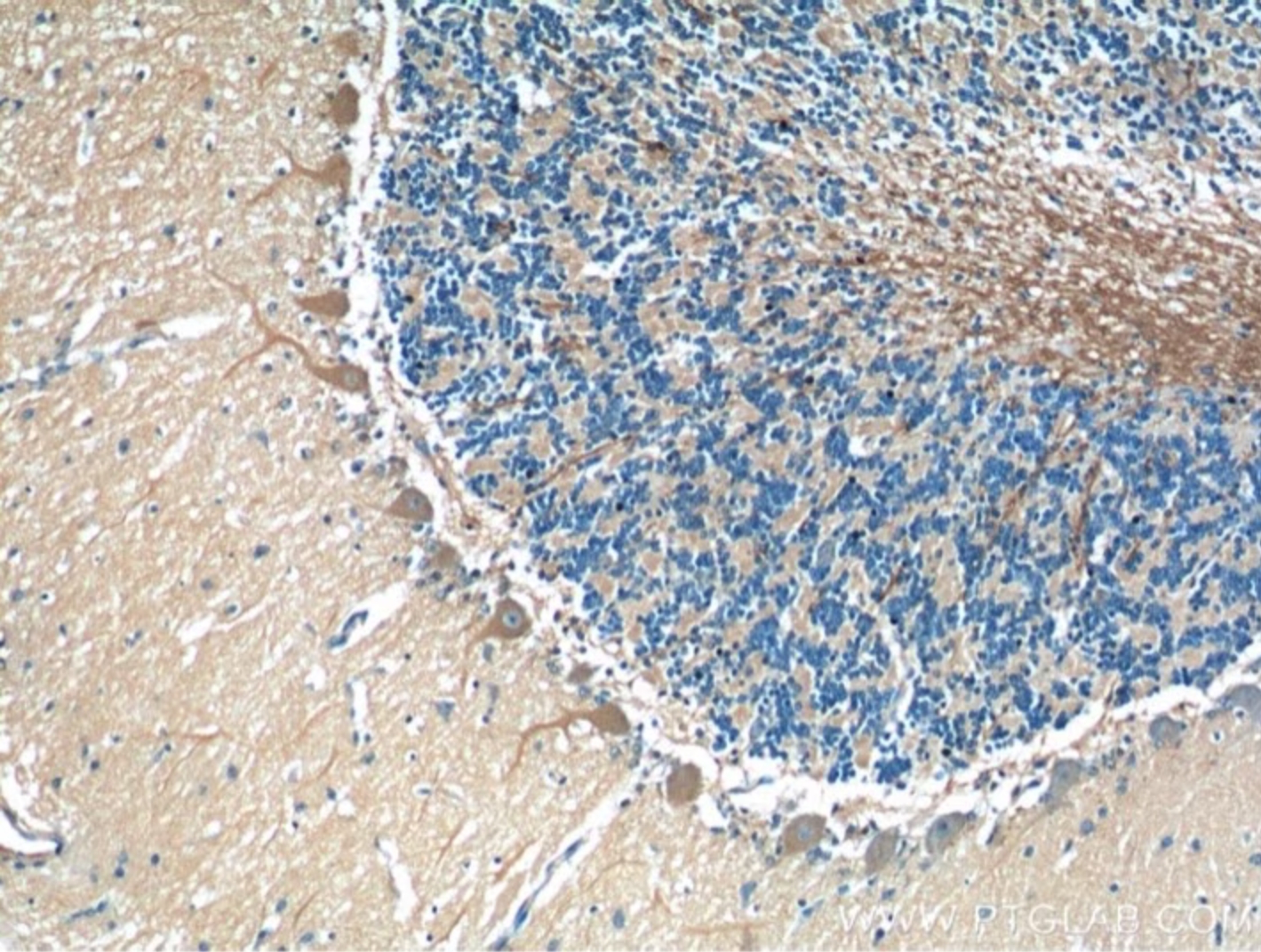
IHC staining of human cerebellum using TMEM106B 60333-1-Ig
Immunohistochemical analysis of paraffin-embedded human cerebellum tissue slide using 60333-1-Ig at dilution of 1:200 (under 10x lens). NB: There are currently no commercial antibodies that can specifically detect the C-terminal fragment as described by Chang et al., Cell and others.
Proteintech’s TDP-43 antibody (10782-2-AP) was used in the discovery of FTD and ALS (Neuman et al. 2006, Science), and since then our TDP-43 antibodies and ELISA kits have been cited over 1600 times. We are incredibly proud of our customers and the work they have contributed to this field, and thus as a company, we are excited by this new discovery. Proteintech has TMEM106B antibodies against the N and C terminus to help researchers further evaluate TMEM106B function.
|
Antibody Name |
Catalog # |
Immunogen |
Notes |
|
20995-1-AP |
C-terminus, 150-274aa
|
siRNA knockdown validated |
|
|
60333-1-Ig |
N-terminus, 1-46aa |
siRNA knockdown validated |
Many questions now need to be asked about TMEM106B’s function in order to capitalize on this important finding. We spoke to scientists whose work is impacted by this discovery and asked them for their opinions and what future research is needed to best understand it.
|
James Quinn, PhD, Research Fellow in Neurology at Massachusetts General Hospital affiliated with Harvard Medical School.
|
“The finding of TMEM106B aggregates in the brains of patients with neurodegenerative diseases including ALS and FTD is extremely interesting, it is not too often in the field that a new protein aggregate is identified in the brains of patients with these diseases as well as in aged brains. However, the link to disease pathogenesis has yet to be fully understood as well as what drives TMEM106B to aggregate and the consequences of this. As a researcher interested in disease biomarkers, I’d be fascinated to see if there are specific forms of TMEM106B that can be identified in the cerebrospinal fluid or plasma from these patients which could be used to track its role in disease progression and for the future if therapeutics targeting TMEM106B are under clinical development.” |
|
Markus Damme, PhD, Research Fellow at the Biochemical Institute, Christian-Albrechts-University, Kiel, Germany
|
“The findings of aggregates and fibrils composed of the luminal domain of TMEM106B are fascinating and provoke a number of new questions that will keep dozens of scientists busy: Where are those fibrils localized? What drives the generation of the luminal domain and fibril-formation? Are the fibrils critical modifiers in patients harboring other dementia risk factors? These questions will help to fully elucidate the contribution of TMEM106b on disease and aging.” |


