7 Tips to successfully culture primary rodent neurons
Proteintech experts provide seven tips for improving the integrity of your primary neuronal cultures.
Primary neuronal cultures are a powerful tool to study cell function, protein interactions, and neurotoxicity, as well as aid in many other in vitro applications. Some of the biggest setbacks in culturing neurons can be obtaining a low cell yield and managing cell death. Optimizing your neuronal preparation workflow can also be time-consuming and costly. Here we provide seven tips for considerably improving the integrity of your primary neuronal cultures.
- Create a matrix with the ideal substrate
- Use embryonic tissue
- Sufficiently dissociate your tissue
- Make sure to use serum-free media with the right supplements
- Know the right density for your experiment
- Work fast
- Let your cultures adapt to their new environment
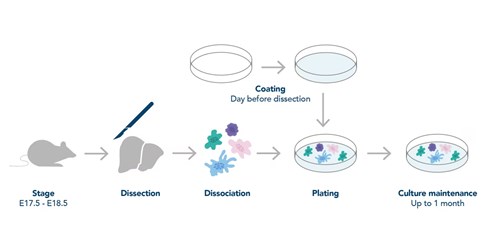
Figure 1. General workflow for neuronal preparation. Figure adapted from Deshpande et al. (2016)
-
Create a matrix with the ideal substrate
Monolayer attachment of neurons to plastic or glass requires extracellular matrix components and other adhesion factors, especially due to their serum-free environment. In order to ensure that your neurons properly adhere, differentiate, and grow in culture, be sure to coat your plates with one or a combination of substrates prior to plating your cells. The most commonly used substrates for neuronal cultures are poly-D-lysine, poly-L-lysine, poly-L-ornithine, fibronectin, collagen, and laminin. In general, poly-D-lysine and poly-L-lysine are used most often. It is important to note that when using poly-L-lysine opt for a higher molecular weight (>MW 30,000–70,000), as shorter polymers can be toxic for your cells. Additionally, ensure that the entire bottom of the well is covered with your substrate to avoid uneven growth and clumping of cells. Prior to plating your neurons, thoroughly wash all excess substrate as residual substrate can be toxic for the neurons.
-
Use embryonic tissue
It is best to use embryonic animals for culturing neurons. Brains from prenatal animals (E16-E18) possess neurons with less extensive processes and connections, making the cells less susceptible to damage during dissociation. In addition, prenatal brains possess many undifferentiated cells. When cultured in the proper media, these cells can differentiate into neurons more easily than cells that have already undergone differentiation. In contrast, the heterogeneity of older brain tissue may contaminate cultures with other cell types such as astrocytes and oligodendrocytes.
-
Sufficiently dissociate tissue
It is important to properly dissociate the neural tissue using a combination of enzymatic, chemical, and mechanical dissociation. If tissue is not properly dissociated, aggregation of cells may occur making it difficult for neurons to form the proper connections in culture. We recommend further chopping neural tissue into smaller pieces after dissection to aid in dissociation. Additionally, consider the enzyme you are using for dissociation. For example, collagenase is gentler than trypsin and may require more time during the dissociation step.
-
Make sure to use serum-free media with the right supplements
The addition of serum in your media will result in improper differentiation of your cells, mainly into astrocytes. The proper media and supplements for culturing neurons are commercially available. In order to avoid the risk of bacterial contamination, be sure to spike your media with the proper antibiotics, such as a penicillin-streptomycin-glutamine mixture. Additionally, when neurons are initially plated, a small amount of L-glutamate (~0.025mM) in the media can aid in neuronal growth and connectivity. However, to avoid cytotoxicity, it is important to be aware of L-glutamate concentration in media and duration of exposure in culture. Finally, to maintain healthy cultures for an extended period, we recommend switching half the culture media with new media every three days.
-
Know the right density for your experiment
Plating neuronal cells at the ideal density is important for the overall health of the culture. Plating cells too densely or too sparsely can cause cell aggregation that generates sphere-like bodies in your culture after only a few days of plating. The general rule of thumb is to plate your cells at a density of about 1,000–5,000 cells per mm2. The ideal density of your culture depends on your experimental question. For example, lower densities are ideal when trying to image individual cells, but higher densities might be required when attempting to western blot for a low-expressed protein.
-
Work fast
As soon as you begin your neuron preparation, the cells are in distress and are at risk of dying! Therefore, it is important to work quickly and efficiently. Ensure you have all the solutions and equipment ready to go prior to beginning your neuron preparation. In addition, consider warming up solutions to avoid extreme temperature changes and placing tissues in a 37°C incubator during enzymatic dissociation to speed up detachment.
-
Let your cultures adapt to their new environment
It might be tempting to check on your cells right after you have plated them, but neurons are very sensitive to their environment. Changes in temperature or agitation can disturb the cells and prevent them from growing. It is best to leave cultures alone as much as possible and only disturb them for media changes and experimental treatments.
Once you have optimized your neuron preparation protocol, you can utilize your cultures for in vitro techniques such as immunocytochemistry, western blotting, ELISA, or flow cytometry. At Proteintech, we offer neuronal marker antibodies for various techniques! Some of these products are also available conjugated to fluorophores or as an IHCeasy kit. The table below includes a few commonly used markers for mature neurons, all available at Proteintech (Table 1).
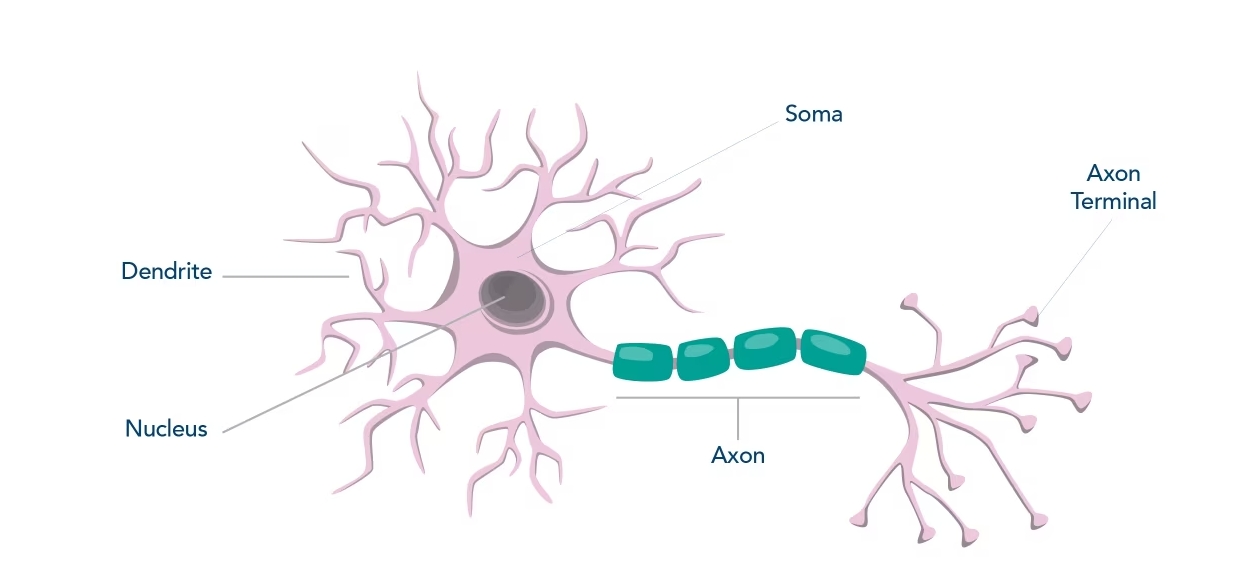
| Nucleus | Soma | Axon | Dendrites | |
| NeuN | X | |||
| MAP2 | X | X | ||
| Tau | X | X | ||
| Beta III Tubulin/ TUBB3 | X | X | X | |
| Enolase 2 | X | |||
| NF-H | X | X | X | |
| NF-M | X | X | X | |
| NF-L | X | X | X |
Table 1. Localization of common neuronal markers for mature neurons.