Breast Cancer Awareness Month
October is an annual health campaign to promote early detection, reduce risk factors and support patients
Introduction:
Breast cancer is a devastating disease and has the highest incidence of all cancers, accounting for 12% of all new cancer diagnoses and 685 000 deaths worldwide in 2020 (World Health Organization). The leading cause of breast cancer deaths is metastasis from the breast to secondary organs such as the bones, liver, brain, and lungs. Much clinical and basic research is focused on improving the early detection of breast cancers and finding more markers of metastasis to improve breast cancer outcomes. Routine screening is well established with mammography, now with the addition of more advanced imaging like Magnetic Resonance Imaging (MRI) and ultrasounds. Breast cancer is compounded by the fact it is a highly heterogenous disease, which can lead to resistance to chemotherapy; however, this can be utilized to allow subtype-specific targeted therapy.
Breast Cancer Subtypes:
The subtypes are based on the tissue of origin within the breast (ductal or lobular), cell of origin (luminal or basal), hormone receptor status (estrogen, progesterone, or HER2 receptor), and whether it is invasive or localized (in situ). These subtypes affect the treatment options, prognosis, and chances of metastatic spread.
Overview of subtypes:
Subtype |
Drug Treatment |
Model Cell Line for Research |
|
Luminal A (ER+/PR+/HER2-) |
Hormone Therapy against ER/PR (e.g., Tamoxifen) |
MCF7, T47D |
|
Luminal B (ER+/PR+/HER+/-) |
Hormone Therapy against ER/PR (e.g., Tamoxifen) |
BT474, MDA-MB-361 |
|
HER2 overexpressing (ER-/PR-/ HER2+) |
HER2 mAb Herceptin |
SKBR3, AU565 |
|
Basal – Triple Negative Breast Cancer (TNBC) (ER-/PR-/HER2-) |
Chemotherapy (Carboplatin, Olaparib) PD-1 inhibitor (Pembroluzimab) |
MDA-MB-468, BT-20 |
|
Non basal – TNBC (Claudin low) |
Chemotherapy (Carboplatin, Olaparib) PD-1 inhibitor (Pembroluzimab) |
MDA-MB-231, BT-549 |
Breast Cancer Stem Cells (BCSCs)
BCSCs represent a small sub-population of breast cancer cells (BCCs) but are highly significant as they are drivers of metastasis and drug resistance, which often results in relapse. The proportion of BCSCs is also highest in the most aggressive basal-like breast cancer subtype. They therefore represent a significant clinical target and major research efforts have contributed to characterizing them. BCSCs are regulated by surface markers, most commonly EpCAM+/Cd44+/CD24-/ALDH1+ and intracellular pathways such as Notch, Wnt/b-Catenin, PI3-K/Akt, and Hedgehog signaling. Methods such as Fluorescent Activated Cell Sorting (FACS) and Magnetic Activated Cell Sorting (MACS) use BCSCs surface markers to isolate and study this cell sub-population. Other research methods include Aldehyde Dehydrogenase assays and spheroid formation assays as a readout of “stemness.”
Check out our Flow Cytometry and Magnetic Cell Separation systems for the isolation of BCSCs.
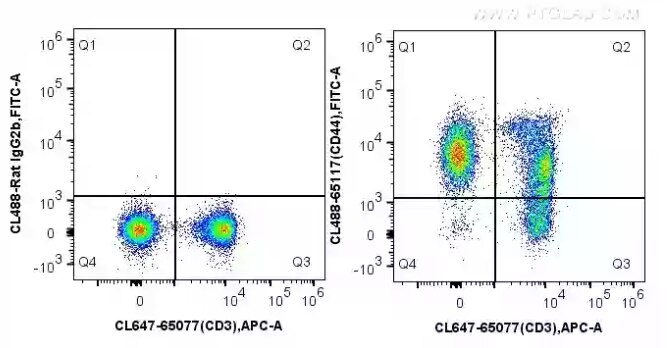
Fig.1 1X10^6 C57BL/6 mouse splenocytes were surface stained with 5 ul CoraLite®647 Anti-Mouse CD3 (17A2) (CL647-65077, Clone: 17A2) and CoraLite®488 conjugated Rat IgG2b (left) or 5 ul CoraLite®488 Anti-Mouse CD44 (CL488-65117, Clone: IM7) (right). Cells were not fixed.
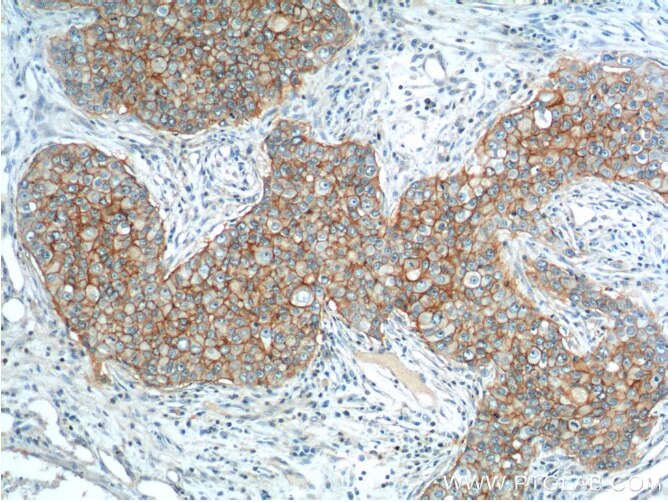
Fig2: Immunohistochemical analysis of paraffin-embedded human breast cancer tissue slide using 21050-1-AP (EPCAM Antibody) at dilution of 1:200 (under 10x lens).
BCSCs are inherently resistant to therapy due to multiple characteristics:
- They are non-proliferative and remain in a G0 cell cycle state, protecting them from the DNA-damaging effects of chemotherapy.
- They overexpress the ATP Binding Cassette (ABC) transporters that use efflux pumps to transport chemotherapeutic agents out of the cell.
- They are often located in the hypoxic region of the tumor that is radioresistant due to lack of oxygen, required for the generation of free oxygen radicals, and is poorly perfused, thus preventing the delivery of other drugs in the circulation.
On the bright side, alternative avenues to target BCSCs have been developed, taking advantage of their specific markers, for example by nanoparticle-mediated therapy. In a nano-delivery system, the nanoparticles target a cell marker (e.g., CD44), enter the cell, and deliver the drug intracellularly. Due to their small size, nanoparticles can address some of the obstacles of standard drug delivery such as solubility, stability, and pharmacokinetic issues. They can also deliver multiple drugs within a single nanoparticle, known as co-encapsulation. Targeting multiple de-regulated pathways simultaneously could prove a very effective way of killing these stem cells, which have high plasticity and self-renewal capability.
Recent Breast Cancer Research Breakthroughs:
- Enhertu – Trastuzumab Deruxtecan (Enhertu®) is a drug with laser precision for HER2 and has been FDA approved as treatment for women with HER2-low breast cancer, further expanding the breast cancer patients who can benefit from targeted therapy: https://www.enhertu.com/ NEJM Paper
- A City of Hope-led trial considered ethnicity as one of the factors when selecting precision medicine options – a huge breakthrough as black women are more likely to develop aggressive forms of breast cancer: https://www.cityofhope.org/clone-endocrine-therapy-surgery-results-different-outcomes-black-vs-white-women-breast-cancer-city. This research was presented last month at the 15th AACR Conference on the science of cancer health disparities in racial/ethnic minorities and the medically underserved.
- TAILORx – Clinical trial focused on which women can safely avoid chemotherapy as part of their breast cancer treatment – moving towards prioritizing targeted therapy for breast cancer rather than as an adjuvant to chemotherapy: https://www.cancer.gov/news-events/press-releases/2018/tailorx-breast-cancer-chemotherapy TAILORx
- Immune targets for chemotherapy-resistant breast cancer identified, expanding the immunotherapy options for those unable to benefit from standard treatments: Paper Press Release
For more information, view our cancer resources page here.
Blog written by Lucie Reboud, Intern at Proteintech, PhD student in cancer research at the University of Manchester.
References:
Song, K., & Farzaneh, M. (2021). Signaling pathways governing breast cancer stem cells behavior. Stem Cell Research & Therapy, 12(1), 1-11.
Zheng, Q., Zhang, M., Zhou, F., Zhang, L., & Meng, X. (2021). The breast cancer stem cells traits and drug resistance. Frontiers in pharmacology, 11, 599965.
Gao, Y., Tang, M., Leung, E., Svirskis, D., Shelling, A., & Wu, Z. (2020). Dual or multiple drug loaded nanoparticles to target breast cancer stem cells. RSC advances, 10(32), 19089-19105.



