Neutralizing antibodies and their role in COVID-19 research
Weathering the Cytokine Storm
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with symptoms such as fever, dry cough, and shortness of breath, has a case fatality rate of ~ 4%, with higher death rates for immunocompromised and elderly individuals. Most patients will develop no or mild symptoms, while some patients may develop hyper-inflammation caused by the production of a large amount of cytokines, known as a cytokine storm, which may lead to fatal pneumonia and acute respiratory distress syndrome (ARDS).
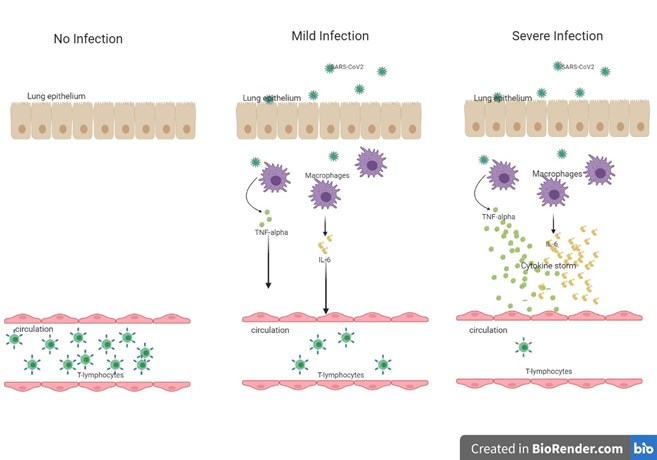
Figure 1. Cytokine storm during SARS-CoV2 Infection
Cytokines or chemokines play a vital role in the appropriate functioning of the immune system. A cytokine storm is a potentially fatal immune reaction with a positive feedback loop between immune cells and cytokines leading to systemic inflammation that can harm tissues, organs, and systems in the body. Higher plasma levels of IL-6, TNF-alpha, and GMCSF, along with IL-10, IL-1B, IL-2, IL-17, 1L-18, and CCL2, have been identified during severe COVID-19 infection (PMID: 31986264). A large amount of these cytokines trigger T-cell lymphopenia, which dampens the adaptive immune response (Figure 1).
Neutralizing antibodies are one way to block the cascade reaction of a cytokine storm by blocking the active sites of these cytokines. Neutralizing antibodies from the blood of recovered patients are being investigated as a possible way to prevent cytokine storms in people currently suffering from the infection. Monoclonal antibody therapies that neutralize IL-6, GMCSF are also being investigated for use along with anti-viral drugs in treating severe cases of COVID-19.
What are neutralizing antibodies?
In the adaptive immune response, antibodies produced by B cells help in combating infection by neutralizing the target so that the host is protected from pathogen invasion. This can happen through a variety of mechanisms such as the blocking pathogen binding to the host or sequestering the pathogen.
Every antibody has a binding affinity for its protein target. For many antibody-based techniques, highly specific binding is sufficient for their use in an experiment such as localization experiments using immunocytochemistry or immunofluorescence, or quantification of protein expression in specimens using western blot.
In certain cases (e.g., cell-based assays or animal studies), antibodies are used to specifically inhibit the biological activity of their target’s protein. This requires an antibody that is not only able to bind to the target but also prevent interactions between the target and other proteins. This can be achieved by occupying binding sites, resulting in a conformational change of the protein target structure or sequestering the target.
Why do scientists use neutralizing antibodies?
Neutralizing antibodies have broad applications for therapy and research. One prominent example is Keytruda, the PD-1 neutralizing antibody used in cancer immunotherapy. In addition, neutralizing antibodies can be used in scientific experiments, such as when verifying the existence of targets and exploring target functions.
It has been reported that a patient with respiratory failure associated with COVID-19 had a rapid favorable result after two infusions of the anti-interleukin 6 receptor inhibitor tocilizumab. This suggests that anti-IL6 receptor inhibitor could reduce the risk of progression to SARS by mitigating a COVID-19 associated cytokine storm (PMID: 32247642). UK-based biotech Izana Bioscience has initiated a study to explore the potential of anti-GMCSF antibody namilumab in the treatment of severe COVID-19 patients. This demonstrates the importance of using neutralizing antibodies for COVID-19 treatment and research.
Introducing Proteintech’s NeutraKine™ series
To explore new areas of biomedical research, scientists need more neutralizing antibodies. However, it can be challenging to generate them. A conventional research antibody can bind to a multitude of sites on an antigen and be an effective tool for detecting proteins in western blot, immunohistochemistry, etc. However, it is difficult to generate an antibody capable of blocking the functionality of an antigen. Additionally, the validation of candidate clones requires the development of a robust in vitro tissue culture assay that has a high degree of variability from test to test. On a related note, the antigen to be neutralized needs to mimic the in vivo counterpart as much as possible, since glycosylations and processing are of critical importance to the function and stability of the protein.
As a manufacturer of HumanKine® cell-expressed cytokines and growth factors, Proteintech has developed in vitro activity assays and uses authentic human proteins as standard. We have leveraged this expertise to launch a line of neutralizing antibodies: the NeutraKine™ series. The key advantages of our process are:
- NeutraKine™ antibodies use authentic HumanKine® human proteins as immunogens, thus more relevant antibodies to natural forms of the target protein are produced.
- NeutraKine™ antibodies are tested against neutralization of HumanKine® protein activity.
Proteintech’s NeutraKine™ neutralizing antibodies are available against multiple cytokines involved in the COVID-19 associated cytokine storm. The blocking of over-produced IL-6 and TNF-alpha during a cytokine storm can be one way of addressing the severity of the disease. NeutraKine™ anti-IL6 and anti-TNF alpha neutralizing antibodies (Figures 2 and 3) offer unparalleled specificity and sensitivity.Proteintech also provides a number of other NeutraKine™ anti-cytokine neutralizing antibodies that could further accelerate the exploration of a universal cure for COVID-19.
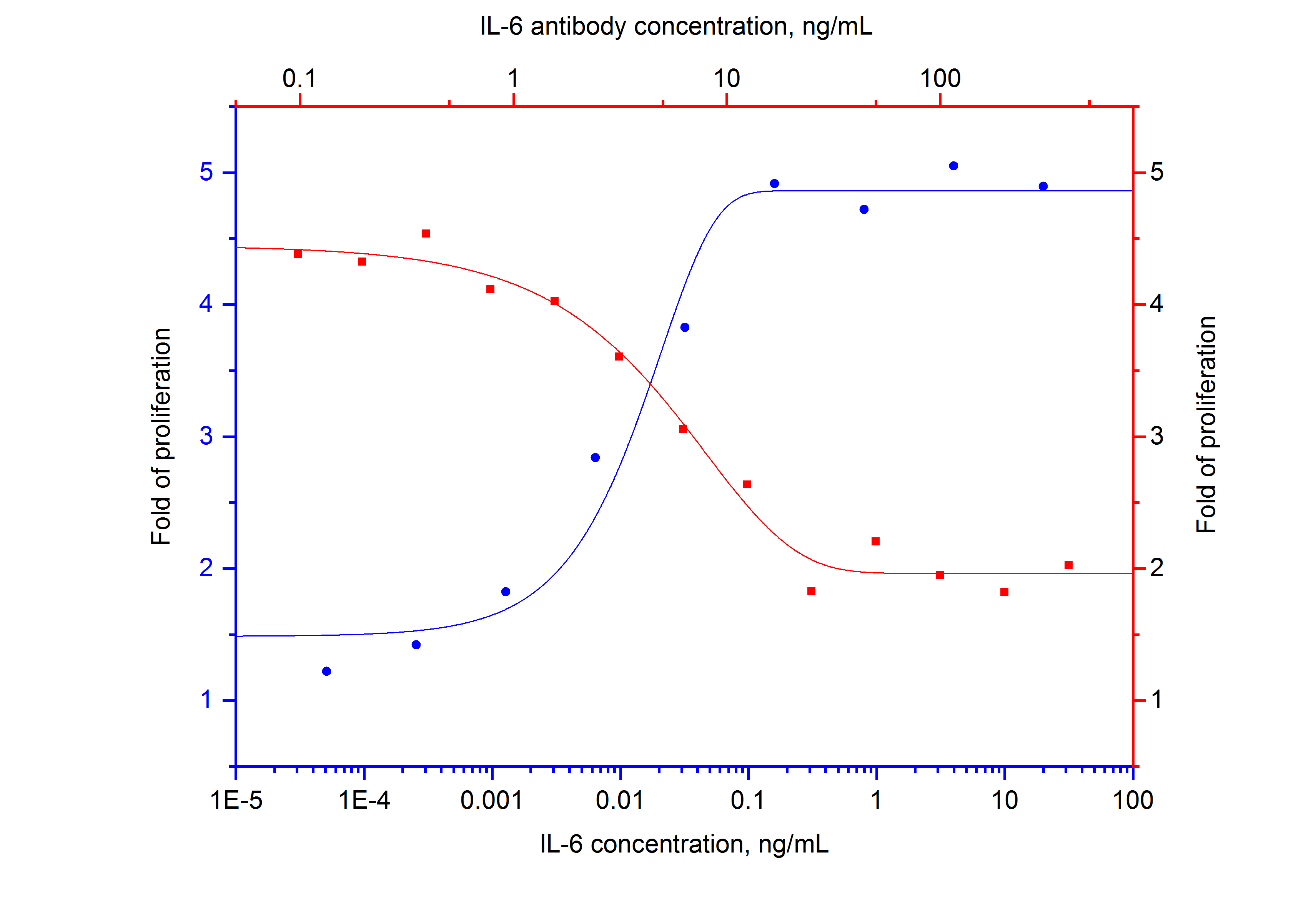
Figure 2. Recombinant HumanKine® IL-6 (HZ-1019) stimulates proliferation of hybridoma (Proteintech anti-GST clone 3G12B10) in a dose-dependent manner (blue curve, refer to the bottom X-axis and left Y-axis). The activity of human IL-6 (1 ng/mL HZ-1019) is neutralized by NeutraKine™ IL-6 antibody (69001-1-IG) at serial dilution (red curve, refer to the top X-axis and right Y-axis). The ND50 is typically 3-15ng/mL.
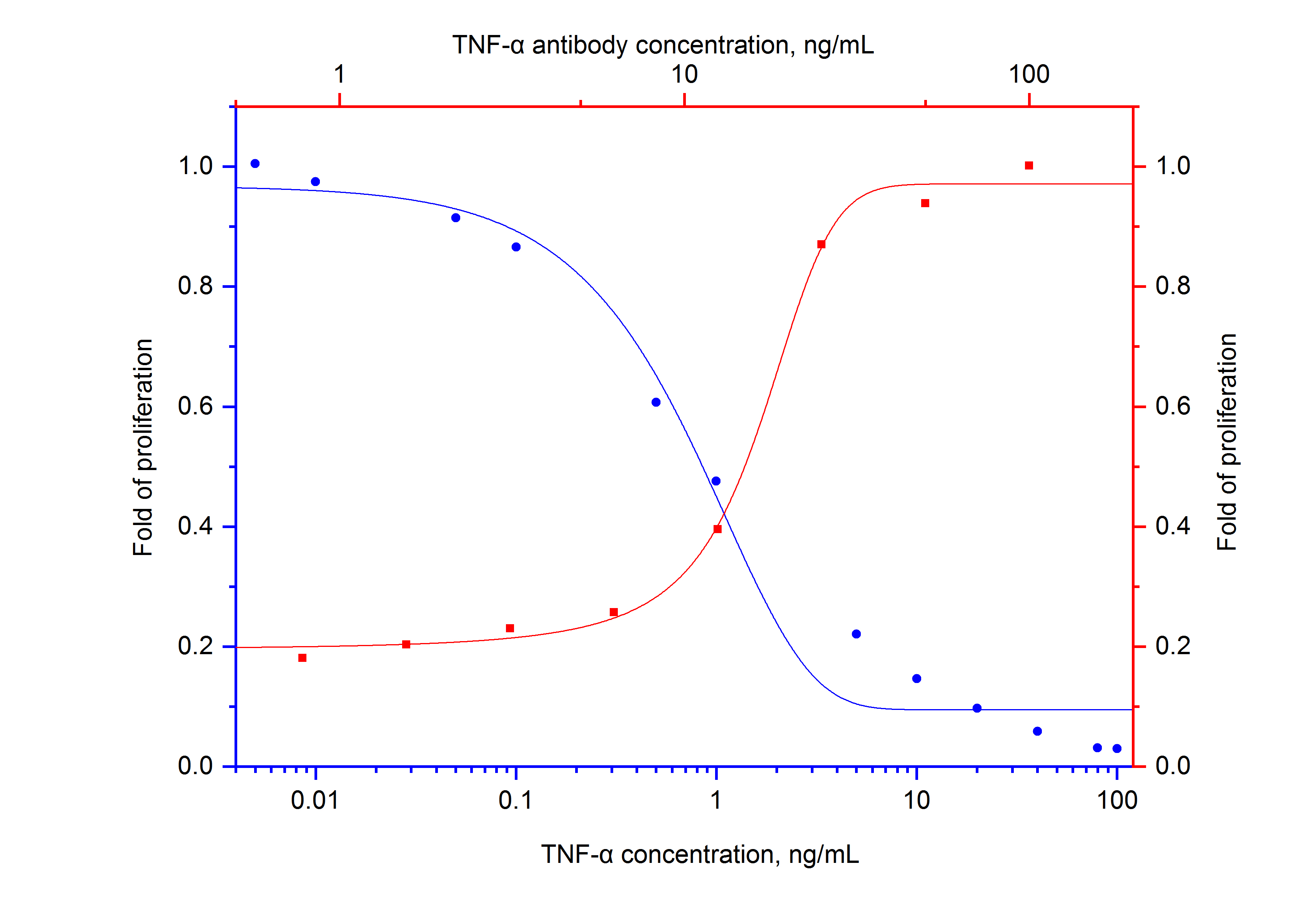
Figure 3. Recombinant Human TNF alpha (HZ-1014) inhibits growth of L-929 cells in a dose-dependent manner (blue curve). The activity elicited by recombinant human TNF-alpha (5 ng/mL) is neutralized by NeutraKine™ anti TNF-alpha antibody (69002-1-IG) at serial dilution (red curve, refer to the top X-axis and right Y-axis). The ND50 is typically 10-40ng/mL.
NeutraKine™ Series Product List
References


